find the electron configuration sc|How to Write the Atomic Orbital Diagram for Scandium (Sc) : Bacolod By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state .
Cutcut Barangay Hall, Leen cor Gail Street, Nepo Subdivision, Cutcut, Angeles City visit us at www.barangaycutcut.comfurther revisions to definitions, pronunciation, etymology, headwords, variant spellings, quotations, and dates; new senses, phrases, and quotations. Revisions and additions of this kind were last incorporated into praetorium, n. in July 2023.
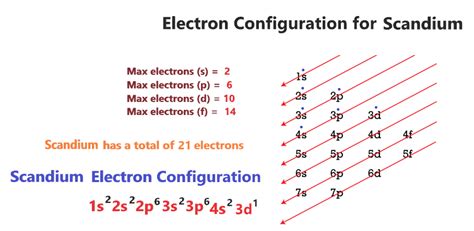
PH0 · Scandium Electron Configuration (Sc) with Orbital Diagram
PH1 · How to Write the Atomic Orbital Diagram for Scandium (Sc)
PH2 · Find the Electron Configuration Sc
PH3 · Electron Configuration for Scandium (Sc, Sc3+ ion)
PH4 · Electron Configuration for Scandium (Sc, Sc3+ ion)
PH5 · Electron Configuration For Scandium
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Electron Configuration Calculator
PH8 · Electron Configuration
PH9 · 2.6: Electron Configurations
EMCEE, QUEZON CITY/CAVITE FACEBOOK / TWITTER / INSTAGRAM. BLKD is an emcee and activist from Cavite and now residing in Quezon City. He started out as a battle rapper on FlipTop, wherein he is regarded as one .PornHub ist die weltweit führende kostenlose Porno-site. Wähle aus Millionen von Harcore Pornovideos, die schnell und in Höchstqualität streamen, sowie VR Pornos. Die umfangreichste Erwachsenen-site des Internets wird immer besser. Wir haben mehr Pornostars und echte Amateure als jede andere site. Schnell, kostenlos, genau das .
find the electron configuration sc*******Mar 23, 2023 Click on above elements (in Periodic table) to see their information or Visit .
Element configuration for atoms or molecules is defined by the number of electrons present in the orbit or shell. In case of Scandium, there are 3 orbits and 17 .Scandium’s electron configuration, 1s² 2s² 2p6 3s² 3p6 3d¹ 4s², reveals how the 21 electrons are distributed among different energy levels and orbitals. Let’s delve into the .
The electron configuration is the arrangement of electrons of an atom. It concerns the way electrons are distributed in the orbitals of the atom. There are 4 4 orbital types ( s s, p p, .
By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state .
To write the orbital diagram for the Scandium atom (Sc) first we need to write the electron configuration for just Sc. To do that we need to find the number of electrons for the Sc atom (there are .find the electron configuration sc How to Write the Atomic Orbital Diagram for Scandium (Sc) When atoms remove 1, 2, or 3 electrons respectively, they will form +1, +2, or +3 ions respectively. Thus, the electrons are removed starting from the highest .
How can you find the electron configuration for an element? How do you find electron configurations for ions? How do you find electron configuration using the periodic table? Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or .To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83).
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.The filling order is based on observed experimental results, and has been confirmed by theoretical calculations.
Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The value of n can be set between 1 to n, where n is the value of the outermost shell containing an electron. This quantum number can only be positive, non-zero, and integer values. That is, n=1,2,3,4,.. For . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait . For example, silicon has nine possible integer oxidation states from −4 to +4, but only -4, 0 and +4 are common oxidation states. Scandium - Electron Configuration and Oxidation States - Sc. Electron configuration of Scandium is [Ar] 3d1 4s2. Possible oxidation states are +3.
In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4.
find the electron configuration scIn several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4. The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital. Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are s-block, groups . Electron configurations of ions. To find the electron configuration for an ion, first identify the configuration for the neutral atom. Then, add or remove electrons depending on the ion's charge. For example, to find the configuration for the lithium ion (Li⁺), start with neutral lithium (1s²2s¹). Then, since the lithium ion has one less .
A step-by-step description of how to write the electron configuration for Scandium (Sc). In order to write the Sc electron configuration we first need to kn.The magnetic form of a substance can be determined by examining its electron configuration: if it shows unpaired electrons, then the substance is paramagnetic; if all electrons are paired, the substance is diamagnetic. This process can be broken into four steps: Find the electron configuration. Draw the valence orbitals. The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .
From Sc on, the 3 d orbitals are actually lower in energy than the 4 s orbital, which means that electrons enter the 3 d orbitals first. In this video, we’ll discuss this in more depth and .

The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium.
Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first.
Pinay Porn Videos: WATCH FREE here! Categories Live Sex Recommended Featured. . Pornkai is a fully automatic search engine for free porn videos. We do not own, produce, or host any of the content on our website. All models were 18 years of .
find the electron configuration sc|How to Write the Atomic Orbital Diagram for Scandium (Sc)